Nicotinic acetylcholine receptors (nAChR) are homo- or hetero-pentameric ligand-gated ion channels of the Cys- loop superfamily that play important roles in the nervous system and muscles such as addiction, pain, memory, cognition, and muscle contraction [1]. Traditional agonists and antagonists of nAChR bind to orthosteric sites on the extracellular domain (ECD). Allosteric ligands of nAChR can bind to the ECD or the transmembrane domain (TMD) to alter the desensitization states of the receptor [2]. Although there has been an increase in the number of available nAChR structures in the last few years, computational modeling still plays an important role in determining interactions between nAChR and their small molecule and peptide ligands due to the flexible nature of nAChR and the existence of multiple receptor states. In the Meiler Lab. our focus is on identification of nAChR – ligand interactions with a specific emphasis on peptide – nAChR interactions. Our work identified key structural motifs that are associated with nAChR activation [3], provided a benchmark for the modeling of peptide toxin – nAChR interactions [4], and led to the identification of the mechanism of action of the only known allosteric agonist peptide ligand of the receptor [5].
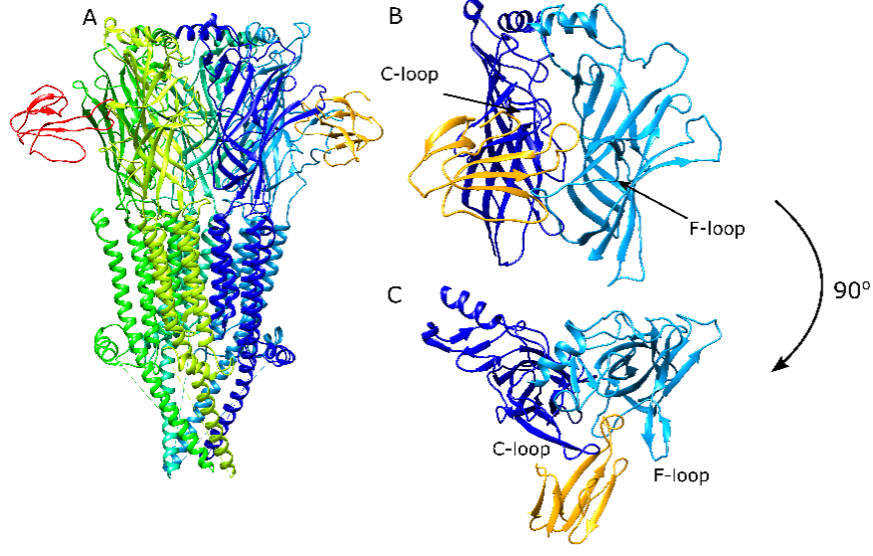
Figure: The structure of the muscle-type Torpedo nAChR and its interactions with a-bungarotoxin viewed from side for the ECD/TMD structure (A), from side of the ECD (B), and from top of the ECD (C).
References:
[1] Changeux, J.P. The nicotinic acetylcholine receptor: The founding father of the pentameric ligand-gated ion channel superfamily. J. Biol. Chem. 2012, 287, 40207–40215, doi:10.1074/jbc.R112.407668.
[2] Gulsevin, A.; Papke, R.L.; Stokes, C.; Garai, S.; Thakur, G.A.; Quadri, M.; Horenstein, N.A. Allosteric agonism of a7 nicotinic acetylcholine receptors: Receptor modulation outside the orthosteric site S. Mol. Pharmacol. 2019, 95, doi:10.1124/mol.119.115758.
[3] Gulsevin, A.; Meiler, J.; Horenstein, N.A. A computational analysis of the factors governing the dynamics of α7 nAChR and its homologues. Biophys. J. 2020, 1–14, doi:10.1016/j.bpj.2020.09.006.
[4] Gulsevin, A.; Meiler, J. An investigation of three-finger toxin-nachr interactions through rosetta protein docking. Toxins (Basel). 2020, 12, doi:10.3390/toxins12090598.
[5] Gulsevin, A.; Papke, R.L.; Stokes, C.; Tran, H.N.T.; Jin, A.H.; Vetter, I.; Meiler, J. The allosteric activation of α7 nAChR by α-conotoxin MrIC is modified by mutations at the vestibular site. bioRxiv Biophys. 2021.
