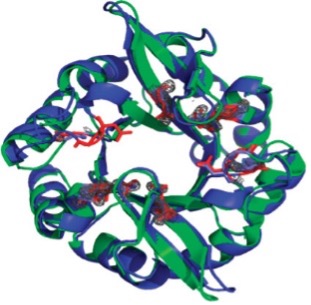
The (βα)8-barrel superfold (TIM barrel) is one of the most frequently observed folds in nature, comprising 10% of proteins with known structures. The domain is composed of eight βα units, linked together by loops which wrap around to form a cylinder of parallel β-strands (β-barrel) surrounded by a layer of parallel α-helices. The wide variety of amino acid sequences that adopt the fold makes it difficult to determine an evolutionary history. This project utilizes one of the most popular hypotheses- the TIM barrel arose through gene duplication and fusion of (βα)2n units. The Meiler lab uses ROSETTA protein design software to computationally engineer symmetric (βα)8 TIM-barrel superfold proteins, then express and characterize these proteins experimentally. One such protein designed and characterized in the Meiler lab, a symmetric variant of imidazole glycerol phosphate synthase termed FLR, adopts the symmetric (βα)8 TIM-barrel superfold. This and other designed proteins are characterized via NMR, x-ray crystallography, and enzymatic activity assays. The assembly of larger proteins from symmetric subunits not only presents an attractive strategy in evolution; it also facilitates the computational design of large proteins, as the symmetry constraint reduces the sequence and conformational search space.